Sanford Antibiotic Guide Free Download
Physician (Sara Cosgrove, M.D., M.S.) and an Infectious Disease pharmacist (Edina Avdic, Pharm.D., M.B.A), the mission of the program is to ensure that every patient at Hopkins on antibiotics gets optimal therapy. These guidelines are a step in that direction. The guidelines were initially developed by Arjun Srinivasan, M.D.. The Skeptics’ Guide to the Universe is produced by SGU Productions, LLC – dedicated to promoting critical thinking, reason, and the public understanding of.
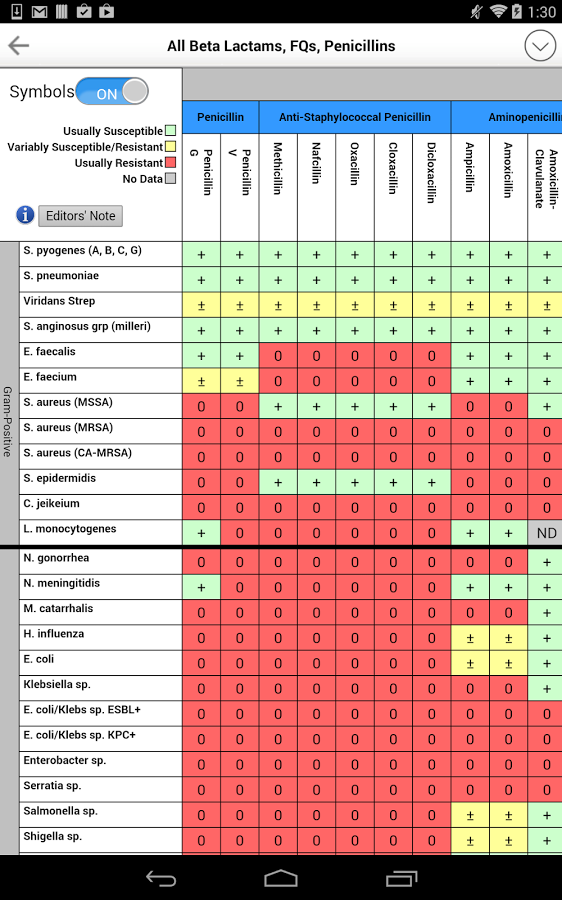
Sanford Guide ID Update features current developments in infectious diseases, curated by the. Links marked with an asterisk (*) provide details to subscribers, while all other links are universal.
If you would like to automatically receive our monthly ID Updates by e-mail,. DECEMBER 2017 New Drug Approvals • *, an INSTI/NNRTI combination formulation of dolutegravir + rilpivirine, is a complete two-drug regimen for treatment of HIV-1 infection in adult patients who 1) are virologically suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen for at least 6 months, 2) have no baseline pre-therapy resistance mutations, and 3) have no history of virologic treatment failure. Standard HIV-1 treatment consists of three or more drugs. • Shingrix (zoster vaccine recombinant, adjuvanted) is a new vaccine indicated for prevention of herpes zoster (shingles) in adults aged 50 years and older. The recommended administration schedule is two 0.5 mL IM doses, given first at month zero and then anytime between 2-6 months later. In late October the Advisory Committee on Immunizations Practices (ACIP) voted that this new vaccine is 1) recommended for healthy adults of age ≥50 to prevent shingles and related complications, 2) recommended for adults who previously received the current vaccine (Zostavax), and 3) the preferred vaccine for preventing shingles and related complications. These recommendations will be published in MMWR and become official policy once they are approved by the CDC director.
First-Time Generic Approvals (US) • Darunavir ethanolate tablets, 600 mg (Teva), approved November 28. • Caspofungin injection, 50 mg and 70 mg vials (Mylan, Gland Pharma), approved September 29. Newly Released Treatment Guidelines • New guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated hemorrhagic cystitis in hematopoietic stem cell transplant recipients from the ECIL-6 (6 th European Conference on Infections in Leukemia) have been published. • Updated guidelines for the diagnosis and management of gastrointestinal complications in adult cancer patients from the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO) have been published.
These guidelines update the 2013 release and are available for download on the. • New European guidelines for the management of pelvic inflammatory disease have been published.
The guidelines are available for download on the International Union against Sexually Transmitted Infections (IUSTI). • Updated guidelines for primary prophylaxis of invasive fungal infections in patients with hematological malignancies from the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO) have been published. These guidelines update the 2014 release and are available for download on the. Sanford Guide Content Integrated into VigiLanz MINNEAPOLIS, MN and SPERRYVILLE, VA. – (November 29, 2017) –, a digital healthcare intelligence firm, announced today that users of its market-leading clinical intelligence platform now have direct access to The Sanford Guide, the industry’s top digital infectious disease content. Integrating content from The Sanford Guide lets clinicians and pharmacists take the critical next step in the antimicrobial de-escalation process by leveraging peer-reviewed recommendations on the latest treatments—without having to exit the VigiLanz workflow.
Through its exclusive partnership with, VigiLanz clients can access the full suite of digital infectious disease content and associate it with specific rules. Users can then search comprehensive coverage of the latest clinician-designed treatment options for infectious diseases, syndromes, and pathogens from within the VigiLanz system, driving improved patient outcomes. “The value of this partnership to our clients extends beyond their VigiLanz users to include any clinician or pharmacist in need of the actionable treatment recommendations, tailored for use in clinical scenarios, from anywhere across the enterprise,” said VigiLanz Chairman and CEO Dr. David Goldsteen, MD, MBA. Sanford Guide ID Update features current developments in infectious diseases, curated by the. Links marked with an asterisk (*) provide details to subscribers, while all other links are universal. If you would like to automatically receive our monthly ID Updates by e-mail,.
NOVEMBER 2017 New Drug Approvals • Heplisav-B, a recombinant, adjuvanted two-dose hepatitis B vaccine, for prevention of infection caused by all known virus subtypes in adults ≥18 years of age. Regimen: two 0.5 mL doses IM, one month apart. • * (Prevymis) for prophylaxis of CMV infection and disease in adult CMV-seropositive recipients of an allogeneic stem cell transplant. The recommended dosage is 480 mg IV or orally once daily, initiated between day 0 and day 28 post-transplant and continued through day 100. Product availability: tablets (240 mg, 480 mg), injection. New Treatment Guidelines • New British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease have been published. The guidelines are available for download on the.
• New clinical practice guidelines for the management of chronic pain in patients living with HIV from the HIV Medicine Association (HIVMA) of the Infectious Diseases Society of America (IDSA) have been published. The guidelines are available for download on the.
Updated Treatment Guidelines • The HHS Panel on Antiretroviral Guidelines for Adults and Adolescents has released an updated version of the Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. The guidelines are available for download on the • The Recommendations for the Use of Antiretroviral Drugs in Pregnant Women with HIV Infection and Interventions to Reduce Perinatal HIV Transmission in the United States have also been updated and are available for download on the. • Updated clinical practice guidelines for the diagnosis and management of infectious diarrhea from the IDSA have been published.
These guidelines update the 2001 release and are available for download on the. • Updated guidelines for the management of * in immunocompetent adults from the Swedish Society of Infectious Diseases have been published.
These guidelines update the 2012 revision. • The Tokyo Guidelines 2018 Antimicrobial Therapy for Acute * have been published.
These guidelines update the 2013 version and are available for download on the of the Journal of Hepato-Biliary-Pancreatic Sciences. Practice Pearls • Tetracycline is generally avoided in young children younger than eight years of age in part because of the risk of teeth staining. However, there are important pharmacologic differences among the members of the tetracycline class so generalizations may be inaccurate. In a retrospective study of 39 children (mean age 0-7.9 years) who received empiric doxycycline therapy (10 mg/kg/day x2-3 days then 5 mg/kg/day, mean treatment duration 12.5 days), no tetracycline-like staining or enamel hypoplasia of developing teeth was detected. Two previous studies likewise found no evidence of teeth discoloration following doxycycline treatment.
• Ciprofloxacin and fluconazole are commonly used for prophylaxis and treatment of infections in patients with hematological malignancies. They are both known for prolonging the QTc interval, a risk factor for the development of potentially lethal ventricular tachyarrhythmias, but how perilous is the combination? In a prospective observational study of 170 patients treated with both drugs, the prevalence of QTc-prolongation (>450 ms in males, >470 ms in females) was low (4.7%, mean prolongation 10.7 ms) and in no patient did the QTc interval exceed 500 ms. No patients experienced complications related to QTc interval prolongation and there were no cases of Torsade de pointes. These data suggest that the QTc-prolonging effect of concomitant ciprofloxacin and fluconazole may not be clinically relevant, and routine ECG monitoring of such patients is probably not necessary. • It is relatively unusual for a beta-lactam antibiotic to affect CYP450 enzymes. Nafcillin induces CYP2C9 and CYP3A4, presumably explaining its ability to decrease the hypoprothrombinemic effect of warfarin.
Similarly, dicloxacillin has been reported to decrease INR in warfarin-treated patients but the mechanism is unestablished. In a study performed in 12 healthy volunteers using a 5-drug pharmacokinetic cocktail, dicloxacillin (1 gm three times daily x10 days) was found to induce CYP2C9, CYP2C19, and CYP3A4. Details of the mechanism were further investigated in vitro using cryopreserved human liver cells. It is reasonable to be cautious when prescribing dicloxacillin to patients receiving narrow therapeutic index drugs metabolized by these CYP enzymes. • The pharmacokinetics of many drugs in patients with cystic fibrosis (CF) are altered relative to healthy persons.
Commonly observed changes include enhanced renal clearance, enhanced hepatic clearance, and variation in volume of distribution. In a prospective study of ceftaroline in seven patients with CF (mean age 20.3 years), the mean half-life was 1. Stihl Serial Number Lookup. 1 hours, considerably shorter than the expected half-life of ceftaroline in the non-CF population (2.7 hours). 15 mg/kg (maximum 600 mg) IV q8h resulted in a mean Cmax of 22.7 µg/mL, which was adequate to achieve >60% time above an MIC of 1 µg/mL (the typical MIC90 of ceftaroline vs. In comparison, 600 mg IV q12h in the non-CF population results in a Cmax of 21.3 µg/mL. These data provide evidence of accelerated clearance of ceftaroline in CF patients and support an increased dosage regimen. Sanford Guide ID Update features current developments in infectious diseases, curated by the. Links marked with an asterisk (*) provide details to subscribers, while all other links are universal.
If you would like to automatically receive our monthly ID Updates by e-mail,. OCTOBER 2017 New Drug Approvals • * (Solosec) for the single-dose treatment of bacterial vaginosis in adult women. The recommended dosage is a 2 gram packet of granules sprinkled onto applesauce, yogurt, or pudding and consumed within 30 minutes without chewing or crunching the granules.
Newly Available Generics (US) • Oseltamivir phosphate oral suspension 6 mg/mL (Nesher Pharmaceuticals), approved September 14. New Treatment Guidelines • New guidelines for the management of * (HAP)/* (VAP) from the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and the Latin American Thoracic Association (ALAT) have been published. The guidelines are available for download on the. Updated Treatment Guidelines • The AASLD-IDSA guidelines for * have been updated. This update (September 21, 2017) reflects several important developments including the recent approvals of * (glecaprevir/pibrentasvir) and * (sofosbuvir/velpatasvir/voxilaprevir).
Trasformare File Da Pdf A Openoffice Templates on this page. While most sections have been updated, the most significant changes have been made in the Initial Treatment of HCV Infection and Retreatment of Persons in Whom Prior Treatment Has Failed sections as well as the Unique Populations sections, which include patients living with HIV, kidney disease, and severe liver disease including those with severe liver dysfunction and those who have had a liver transplant. The updated guidelines are available at, a website developed by the AASLD and the IDSA to provide up-to-date guidance on the treatment of hepatitis C. Antimicrobial Stewardship • In patients with acute respiratory infection, does greater certainly about etiology improve physician prescribing? In a pragmatic, open-label, randomized trial, patients seen in the ED or acute medical unit of a large hospital in the UK were assigned to receive point-of-care testing (POCT) or routine clinical care. POCT consisted of a rapid molecular test for 15 respiratory viruses that provided results within a few hours. No difference was observed in the proportion of patients receiving antibiotics in each group (83%), although more than half the patients in the POCT group received antibiotics before test results were available, and mean duration of antibiotics was also the same in the two groups (about seven days). However, significantly more patients in the POCT group received only one dose of antibiotics than patients in the routine care group (10% vs. 3%), and duration of treatment.
VigiLanz Becomes First Clinical Intelligence Platform to Integrate Sanford Guide Infectious Disease Content MINNEAPOLIS, MN and SPERRYVILLE, VA. – (September 26, 2017) –, a digital healthcare intelligence firm, and, publisher and developer of The Sanford Guide, announced today an exclusive partnership that brings together the market-leading clinical intelligence platform and the industry’s top digital infectious disease content.
Doing so provides VigiLanz users with the critical next step in the clinical surveillance process—access to peer-reviewed recommendations on the latest treatments for improved patient outcomes. The agreement with Sanford Guide, the most trusted name in the treatment of infectious disease, is just the latest in a string of achievements for VigiLanz.
Most recently, its antimicrobial stewardship platform achieved the top overall satisfaction rating in the “” performance report from KLAS Research. The VigiLanz clinical intelligence platform leverages real-time monitoring of thousands of patient care data elements to help improve medication safety, antimicrobial stewardship, infection prevention, quality and care management, and patient safety. Its state-of-the-art rules engine analyzes that data and pushes alerts to clinicians at the point of care when interventions and other actions are necessary, driving significant improvements in safety, efficiency, performance, and reimbursement. “Integrating Sanford Guide content into this process answers the question, ‘What’s next in the antimicrobial de-escalation process?’ by giving clinicians and pharmacists convenient access to the latest clinician-designed treatment recommendations without having to exit the VigiLanz system,” said VigiLanz Chairman and CEO Dr. David Goldsteen, MD, MBA. “We are proud to provide our clients with access to The Sanford Guide’s exceptional content and clinical guidance, more so because we are the first and only clinical intelligence platform able to do so.” Trusted by clinicians in more than 100 countries, The Sanford Guide is optimized to minimize time-to-answer while providing comprehensive guidance at the point of care. Edited by distinguished infectious disease experts from leading academic and clinical centers, Sanford Guide content provides comprehensive coverage of treatment options for infectious diseases, syndromes, and pathogens.
Under the partnership agreement, VigiLanz clients will have access to the full suite of Sanford Guide digital infectious disease content. With continually updated information based on the latest available evidence, focused anti-infective drug information, interactive dosing tables, and extensive links to references and related resources, The Sanford Guide provides actionable guidance that is accessible, concise, and reliable. “This partnership with VigiLanz is the most unique collaboration our company has undertaken since its founding nearly 50 years ago. As we moved forward with ever more sophisticated digital offerings, it was imperative that the company we aligned with share our values and commitment to improving the quality of treatment through focused clinical guidance at the point of care. VigiLanz was that company,” said Sanford Guide CEO and Managing Editor Jeb Sanford.
“Together we have created a product that fully integrates trusted clinical treatment recommendations with top-rated clinical surveillance tools. Our goal is to improve patient outcomes while minimizing technology burdens on providers.” For more information on the VigiLanz-Sanford Guide partnership, visit VigiLanz in booth #226 or Sanford Guide in booth #710 during, taking place October 4-8 in San Diego, CA. About Sanford Guide Since 1969, Sanford Guide has been a leader in point-of-care recommendations for the treatment of infectious diseases. Widely used by pharmacists, physicians, physician assistants, and nurses, Sanford Guide helps to improve patient care by providing carefully curated recommendations based on the latest evidence. With over 1 million users worldwide, Sanford Guide takes pride in responsiveness to customers, the development of innovative solutions, and providing content that is unparalleled in quality and clinical applicability. About VigiLanz Founded in 2001, VigiLanz Corporation (www.vigilanzcorp.com) is a privately held, rapidly growing provider of SaaS health care intelligence and predictive analytics. The firm is focused on aggregating disparate EHR transactional workflow and documentation data across health systems to identify real-time clinical issues that avoid or minimize harm, optimize clinical outcomes and support preventive care along the entire health system continuum. VigiLanz supports a large and growing community of hospital CMOs, CMIOs, CIOs, quality teams, infectious disease and control specialists, pharmacists, and other clinicians dedicated to real-time inpatient and outpatient care.
VigiLanz is shaping the emerging era of real-time health care by delivering enterprise intelligence technology and services that improve clinical outcomes, patient care and operational effectiveness.